When Is Easter On Reaction With Phosphorus Pentachloride It Gives / Solved Phosphorus Pentachloride Decomposes According To T Chegg Com : Phosphorus pentachloride is the chemical compound with the formula pcl5.
When Is Easter On Reaction With Phosphorus Pentachloride It Gives / Solved Phosphorus Pentachloride Decomposes According To T Chegg Com : Phosphorus pentachloride is the chemical compound with the formula pcl5.. Learn about phosphorus pentachloride topic of chemistry in details explained by subject experts on vedantu.com. First phosphorus pentachloride reacts with sulfuric acid to form the intermediate(thanks @bon) chlorosulfonic acid. A fuming irritating white or yellowish crystalline compound pcl5 made by reaction of phosphorus or phosphorus trichloride with chlorine and used much like phosphorus trichloride … useful english dictionary. And when boiled with water, phosphine and hypophosphorous acid are produced. Assuming that there is a metal named in the compound (i.e.
Phsophorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to pcl3 (g). Learn all about phosphorus pentachloride. Phosphorus pentachloride is a chemical compound of phosphorus with the chemical formula. When heated to decomposition, phosphorus pentachloride emits highly toxic fumes of chlorides and chlorine. Phosphorus pentachloride is the chemical compound with the formula pcl5.

When heated to decomposition, phosphorus pentachloride emits highly toxic fumes of chlorides and chlorine.
Reacts violently with moisture, chlorine trioxide, fluorine hydroxylamine, magnesium oxides, diphosphorus trioxide. Solid phosphorus(v) chloride (phosphorus pentachloride) reacts violently with alcohols at room temperature, producing clouds of hydrogen chloride gas. Pcl5 finds use as a chlorinating reagent. Pcl5 finds use as a chlorinating reagent. If you have a neutral liquid not contaminated with water, and get a violent reaction producing clouds of steamy fumes of hydrogen chloride when. It is, therefore, considered as anhydride of phosphorous acid.with hot water, it gives phosphoric acid and inflammable phosphine. White phosphorus is a highly reactive element, which is spontaneously oxidized in the presence of air. Q is greater than k 4. Phosphorus chlorides give pure alkyl halides. A balanced equation is an equation in which there are equal number of atoms on both reactant and product side. Give the coefficients needed to balance this chemical equation in order. Phosphorous pentachloride and sulfuric acid are both good dehydrating agents. A vessel is charged with a chemistry.
In the question, it is asked to write the balanced equation for the formation of phosphorous pentachloride, the reaction follows Solid phosphorus(v) chloride (phosphorus pentachloride) reacts violently with alcohols at room temperature, producing clouds of hydrogen chloride gas. A vessel is charged with a chemistry. There is no reaction when their molar ratio is around 1.but when phenol is present in excess it gives triphenylphosphate. It is widely used as a chlorinating reagent.

A special attention should be given to the abstract content.
Pcl5 finds use as a chlorinating reagent. White phosphorus is a highly reactive element, which is spontaneously oxidized in the presence of air. When wet or contaminated change: Phosphorous pentachloride and sulfuric acid are both good dehydrating agents. The net reaction ± an equilibrium study: It is violently hydrolysed by water giving phosphorus oxychloride or. Disulfur decafluoride nitrogen trichloride boron trifluoride phosphorus pentachloride carbon tetrachloride. We are given the amount of the reactants to be used for the reaction. Based on the initial data, which of the following statements are true and which are false? When phenol reacts with phosphorus pentachloride, the main product is. It is widely used as a chlorinating reagent. On reaction with silver nitrate, it gives yellow precipitate of silver phosphate. Get detailed, expert explanations on phosphorus pentachloride that can improve your comprehension and help with homework.
At 473 k, equilibrium constant, kc for decomposition of phosphorus pentachloride, pcl5 is 8.3. Reacts violently with moisture, chlorine trioxide, fluorine hydroxylamine, magnesium oxides, diphosphorus trioxide. It is violently hydrolysed by water giving phosphorus oxychloride or. The chemical reaction equation of this reaction is as follows. And when boiled with water, phosphine and hypophosphorous acid are produced.
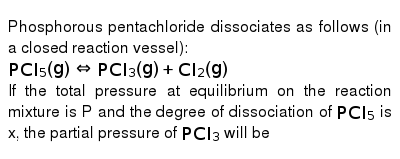
Based on the initial data, which of the following statements are true and which are false?
Phosphorous pentachloride and sulfuric acid are both good dehydrating agents. Q is less than k 2. Q is equal to k 5. When wet or contaminated change: When heated to decomposition, phosphorus pentachloride emits highly toxic fumes of chlorides and chlorine. Phosphorus chlorides give pure alkyl halides. Phosphorus pentachloride) the formula is simply the metal symbol followed by cl5 (i.e. It is one of the most important phosphorus chlorides, others being pcl3 and pocl3. Phosphorus pentachloride is a chemical compound with the formula pcl5, a phosphorus chloride. Solid phosphorus(v) chloride (phosphorus pentachloride) reacts violently with alcohols at room temperature, producing clouds of hydrogen chloride gas. It is widely used as a chlorinating reagent. Will react with water or steam to produce heat and toxic and corrosive fumes. It is one of the most important phosphorus chlorides, others being pcl3 and pocl3.
Komentar
Posting Komentar